Abstract
Introduction
The tumor microenvironment (TME) plays a crucial role in therapy response and modulation of immunologic surveillance. Adjuvant immunotherapy has recently been introduced in post-surgery treatment of locally advanced esophageal cancer (EC) with residual pathological disease after neoadjuvant chemoradiotherapy (nCRT). F-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) remains a valuable imaging tool to assess therapy response and to visualize metabolic TME; however, there is still a paucity in understanding the interaction between the TME and nCRT response. This systematic review investigated the potential of TME biomarkers and 18F-FDG-PET/CT features to predict pathological and clinical response (CR) after nCRT in EC.
Methods
A literature search of the Medline and Embase electronic databases identified 4190 studies. Studies regarding immune and metabolic TME biomarkers and 18F-FDG-PET/CT features were included for predicting pathological response (PR) and/or CR after nCRT. Separate analyses were performed for 18F-FDG-PET/CT markers and these TME biomarkers.
Results
The final analysis included 21 studies—10 about immune and metabolic markers alone and 11 with additional 18F-FDG-PET/CT features. High CD8 infiltration before and after nCRT, and CD3 and CD4 infiltration after nCRT, generally correlated with better PR. A high expression of tumoral or stromal programmed death-ligand 1 (PD-L1) after nCRT was generally associated with poor PR. Moreover, total lesion glycolysis (TLG) and metabolic tumor volume (MTV) of the primary tumor were potentially predictive for clinical and PR.
Conclusion
CD8, CD4, CD3, and PD-L1 are promising immune markers in predicting PR, whereas TLG and MTV are potential 18F-FDG-PET/CT features to predict clinical and PR after nCRT in EC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Standard curative treatment for potentially resectable locally advanced esophageal cancer (EC) (clinical TNM classification cT2-4a/Nany/M0) consists of neoadjuvant chemoradiotherapy (nCRT) followed by surgery according to the CROSS regimen.1 Although 20–30% of patients achieved a pathologic complete response (pCR), more than half still have residual tumor after nCRT.2 The tumor microenvironment (TME) is currently a focus in exploring additional treatment combinations. Several potential predictive markers have been evaluated to improve treatment outcome, including the expression of human epidermal growth factor receptor 2 (HER2) and programmed death-ligand 1 (PD-L1), as well as the prevalence of microsatellite instability (MSI). Based on the presence of MSI and PD-L1 expression among squamous cell (ESCC) and adenocarcinoma (EAC) of the esophagus, including the results of the landmark CheckMate-577 trial, adjuvant immunotherapy has been suggested in EC patients with residual pathologic disease.3,4,5,6,7,8,9,10 Differences in response of only immune checkpoint inhibitors have been observed in ESCC and EAC, which might be associated with important differences in the TME in upper/mid-esophageal (ESCC) and lower esophagus/gastroesophageal adenocarcinoma (EAC/GEA).11,12,13 Compared with EAC/GEA, ESCC exhibited a high expression of PD-L1 and a low HER-2 expression and high MSI (MSI-H) status.14
Therapy response and the activities of the TME are commonly visualized with F-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) scanning. 18F-FDG-uptake (glucose analog) measured by PET/CT indicates the highly increased glucose uptake because of the Warburg effect in tumor tissue. Many studies showed that the increased uptake of glucose and glycolysis by esophageal tumor cells might be caused by enhancement of membrane-bound glucose transporters (GLUT) and hexokinase (HK) enzymes.15,16,17 Studies have ever since tried to associate the Warburg effect in the tumor and its increased TME metabolic biomarkers with the semiquantitative standardized maximum uptake value (SUVmax) in 18F-FDG-PET/CT.18
However, there is still a gap in our understanding of how the TME interacts with nCRT in EC. Therefore, we performed a systematic review to explore potential metabolic and immune TME biomarkers and their predictive role in pathological response (PR) and/or clinical response (CR) after nCRT in EC. As 18F-FDG-PET/CT may visualize the metabolic activity throughout the entire tumor, including its inflammatory microenvironment, it can be used to study the effect of additional immunotherapy in future studies. Combined with potent biomarkers, this metabolic imaging may be helpful in determining response to identify patients more likely to benefit from additional treatment or a potentially applicable organ-preserving treatment approach. Therefore, we also aimed to provide some future research perspectives on metabolic and immune TME biomarkers that might be associated with 18F-FDG-PET/CT (semi)-quantitative features.
Materials and Methods
Search Strategy and Study Selection Process
A systematic review according to the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) guidelines was performed.19 The study protocol was registered and the search strategy was documented online at the International Prospective Register of Systematic Reviews Registry (PROSPERO; ID CRD42022325532). The research question was to explore potential predictive immune and metabolic biomarkers in the interaction of nCRT and TME for a more effective treatment strategy. The exact search strategy is provided in electronic supplementary material (ESM) Table 1. The EMBASE and PubMed online databases were searched from 2001 until September 2022 using the following inclusion criteria: (1) original article/conference abstracts; (2) studies on ESCC or EAC and/or GEA; (3) published in peer-reviewed journals from 2001 or later; (4) studies on the effect of the metabolic, immune and PET-based TME on PR and/or CR after neoadjuvant treatment; and (5) studies published in English. The exclusion criteria were (1) studies with missing or unclear description/criteria for groups and/or variables; (2) if full text was not available; (3) studies not assessing CR after nCRT on pre- and post-treatment PET/CT; and (4) studies not including pathologic reports of the esophageal biopsy and PR of the surgical resection material.
Quality Assessment
Risk of bias was assessed according to the study design and purpose. Non-randomized intervention studies were assessed using the Cochrane Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool.20 All studies were evaluated with a visualization tool for risk-of-bias assessments in a systematic review (Risk-of-Bias VISualization Tool). Each article was read and assessed by two independent authors (HHW, ENS).
Data Extraction and Synthesis
Two authors (HHW, ENS) extracted the data independently. Disagreements between individual judgments were resolved by discussion among the research group consisting of two surgical oncologists, one medical oncologist and one pathologist (all experienced) until consensus was reached. Data were recorded, extracted and managed in a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA). The extraction and generation of the results were discussed together with a statistician (JGMB).
Relative and percentage ΔSUV, total lesion glycolysis (TLG) and metabolic tumor volume (MTV) changes were considered to be an index for CR on 18F-FDG-PET/CT scans.
Results
Identification of Studies
The initial electronic search identified 4190 studies. After eliminating duplicates, 3097 studies remained. These studies were screened using title and/or abstract to assess relevancy to our study scope. As both PR and CR were assessed, we distinguished between studies that included 18F-FDG-PET/CT scans and studies that did not. Seventy-eight articles were included for full screening (31 congress abstracts, 47 original articles); 57 were excluded due to unclear description/criteria for groups and/or variables (n = 34) or studies that did not assess PR and/or CR (n = 23). Finally, we included 21 studies (20 original articles21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40 and one study congress abstract41). We identified 10 studies on biological immune and metabolic TME biomarkers without the presence of an 18F-FDG-PET/CT scan (two studies on metabolic biomarkers, eight on immune biomarkers). Eleven studies were considered significant on clinical immune and metabolic TME biomarkers with the presence of an 18F-FDG-PET/CT scan (10 studies on metabolic biomarkers and 1 study on immune biomarkers) (Fig. 1).
Screening of articles according to the PRISMA flowchart. EAC esophageal adenocarcinoma, ESCC esophageal squamous cell carcinoma, PR pathologic response, CR clinical response, nCRT neoadjuvant chemoradiotherapy, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, 18F-FDG-PET/CT F-18 fluorodeoxyglucose positron emission tomography/computed tomography
Study Characteristics
An overview of the study characteristics of the selected studies is provided in Table 1. Three studies26,32,37 were prospective and 18 studies were retrospective.21,22,23,24,26,27,28,29,30,31,33,34,35,36, 38,39,40,41 Eight studies assessed only EAC,24,26,27,28,30, 34,35,40 six studies assessed only ESCC,21,23,25,36,37,38 and seven studies included both types.18,19,20,21,27,35,37
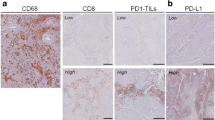
All 21 studies assessed PR, of which 9 studies used the Mandard TRG scoring system,23,24,26,27,28,30,32, 33,36 9 studies only assessed whether pCR was achieved21,22,25,31,34,35, 37,38,41 (defined as no viable tumor cells; ypT0), one study used the assessment of the College of American Pathologist (1–3),29 1 study used pathologic grading according to Schneider et al.,39,42 and 1 study used pathologic grading according to Chirieac et al.43 Eight studies assessed immune markers, of which PD-L1, PD-1, CD80, CD8, CD4 and CD3 were assessed most extensively.23,24,25,26,27,28,29,30 Three studies assessed CD8, PD-L1 and PD-1 in diagnostic tumor biopsies before nCRT,24,27,28 while six studies assessed CD4, CD8, PD-L1, PD-1, CD80 and CD3 in surgical resection specimens after nCRT.23,24,25,26,29,30
Two studies determined whether diabetes mellitus (DM) affected pathologic outcome.31,41 In one of these studies, diabetic and non-diabetic patients were matched on patient and tumor characteristics.31 Both studies included both type 1 and type 2 DM.
All studies on CR included a baseline and post-nCRT 18F-FDG-PET scan. Eleven studies assessed CR,21,22,32,33,34,35,36,37,38,39,40 of which six studies assessed ΔSUVmax,21,32,34,35,38, 40 four studies assessed percentage reduction SUVmax,34, 37,38,39 four studies assessed mean tumor volume (MTV),32, 33,36,40 two studies assessed tumor lesion glycolysis (TLG),36,40 and three studies assessed ΔSUVmean.32,33,34
Effect of Metabolic Markers on Pathologic Response
Two studies on the effect of DM on pathologic response were included and are shown in Table 2. In total, 73 diabetic patients and 293 non-diabetic patients were included. DM was associated with a decreased likelihood of achieving pCR according to Alvarado et al.,31 whereas Boyd et al. showed no significant difference between both groups.41
Effect of Immune Markers on Pathologic Response
Tables 3 and 4 show the pathologic immune markers on PR in treatment-naïve biopsies (Table 3) and surgical resections after nCRT (Table 4) in the primary tumor/TME/overall tumor area. Treatment-naïve biopsies were collected and assessed on immune markers prior to nCRT. The median density of immune markers was assessed in the total area.
As the included studies combined different TRG groups, we were unable to create consistent TRG groups for this review. Tumor regression in these studies was based on vital tumor tissue at the ratio of fibrosis. In addition, patients with pCR (TRG1) were considered free of residual tumor, which is less likely compared with those with non-pCR (TRG2–5). Therefore treatment-naïve biopsies (Table 3) were divided according to the pathologic examination of the resected specimen in good (TRG1–3) and poor (TRG4–5) responders. The Mandard response rates from the treatment-naïve biopsies were extrapolated from their resected specimens. In assessing potential biomarkers in the resected specimen (Table 4), responders after nCRT were divided into pathologic good responders (TRG1–2) and pathologic poor responders (TRG3–5).
Table 3 shows that an overall higher tumoral and TME infiltration of CD8 in treatment-naïve biopsies was associated with a better PR (p = 0.013 and p = 0.026; p = 0.001; p = 0.031, respectively)24,27 Moreover, a higher PD-1 in the TME seemed to significantly predict the possible poor response in tumor tissue from treatment-naïve biopsies (p = 0.048) (Table 4); however, PD-1 in the primary tumor was shown to not be predictive for tumor response (p = 0.222) (Table 3).27,28 PD-L1 expression in the treatment-naïve biopsies showed to predict better PR (lower TRG) both in the TME as the overall tumoral and the TME area (p = 0.036, p = 0.010, respectively).25,27 Only Huang et al. showed that a high density of PD-L1 in the treatment-naïve biopsies predicted poor PR (higher TRG) (p = 0.036).25
Table 4 shows that tumoral and stromal CD8 was found to be significantly higher in pathologic good responders as well as in the healthy mucosa in resected specimens after nCRT.26,44,45,46 Soeratram et al., who distinguished tumoral and stromal CD8, showed that stromal CD8 was significantly associated with good pathologic response (p = 0.000, whereas tumoral CD8 was correlated with a poorer pathologic response (p = 0.000).27 Koemans et al. showed that good responders had significantly less CD8 in the overall area compared with poor responders after nCRT (p = 0.001).30
The majority of the studies found significant enrichment of CD4 in the tumor and the TME in surgical resection specimens after CRT (p = 0.006, p = 0.009, p = 0.004, respectively) (Table 4);23,29 however, one study contradicted these results and showed that poor responders had significant enrichment of CD4 density compared with poor responders (p ≤ 0.001).30
Furthermore, higher PD-1 in the overall tumor and stromal area was shown to be significantly predictive for a poor PR after nCRT (p = 0.0065).26
PD-L1 expression after nCRT proved to be associated with a poor PR according to Koemans et al. (p = 0.001).30 Moreover, a high PD-L1 in the overall area was correlated with a poor PR after nCRT (p = 0.0005, p = 0.010, respectively).26,27
Regarding CD80, two studies revealed no differences in CD80 between pathologic good and poor responders in the overall tumoral and stromal area after nCRT (p = 0.4874, p = 0.89, respectively).26,44
Effect of Clinical Metabolic Markers on Pathological Response
We considered the semi-quantative tools that are used for measuring glucose metabolism and 18F-FDG uptake (SUVmax, SUVmean, ΔSUVmax and percentage reduction SUVmax) in the 18F-FDG-PET/CT scan as clinical metabolic markers. Table 5 describes the effect of ΔSUVmax, percentage reduction SUVmax, ΔSUVmean, TLG, MTV, and ΔSUVratio on pathologic response. Pathologic responders were divided into good responders (TRG1–2) and poor responders (TRG3–5).
ΔSUVmax was evaluated in six studies.21,32,34, 35,38,40 Kukar et al. and van Rossum et al. showed that ΔSUVmax was higher in pathologic good responders (p = 0.03, p = 0.01, respectively).34,40 Moreover, Li et al. assessed ΔSUVmax as an independent predictor for pCR (p = 0.002).21 However, Arnett et al. and Lee et al. found no significant difference between ΔSUVmax in good and poor responders.38,47
Four studies assessed the effect of percentage reduction SUVmax,34,37,38,39 of which two showed no significant difference between pathologic good and poor responders.38,48 Kukar et al. showed that pathologic good responders had a higher percentage reduction SUVmax,34 while Dewan et al. set a cut-off of 72.32% reduction of SUVmax to be predictive for pCR.37
TLG was evaluated in two studies, showing that a high TLG before and after CRT was associated with poor PR (p = 0.0318, p = 0.01, respectively).36,40
Four studies assessed the effect of MTV,32,33,36,40 of which two showed that a high post-CRT MTV was correlated with a poor PR (p = 0.0005, p = 0.01, respectively).36,40 The other two studies showed no correlation with PR (p = 0.6, p = 0.472, respectively).32,33
ΔSUVmean was assessed in three studies, of which two showed no correlation between pathologic response.32,33,34 However, Kukar et al. assessed that pathologic good responders had a higher ΔSUVmean compared with poor responders (p = 0.03).34
Only one study evaluated body mass index on PR, which showed no significant prediction for pCR (p = 0.9879).22
Effect of Metabolic and Immune Markers on Clinical Response and Pathologic Response (18F-FDG-PET/CT)
ESM Table 2 shows the effect of immune and metabolic markers on PR and CR. Both studies divided the assessed groups into pCR (TRG1) or no pCR (TRG2–5).
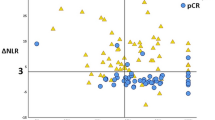
Wang et al. evaluated the effect of obesity as a metabolic marker on CR, which showed not to be a significant predictor (p = 0.46).22 Li et al. assessed the correlation between immune markers neutrophil to lymphocyte ratio (NLR) and PET markers on prediction of PR, which showed that ΔNLR <3 and ΔSUVratio >58% gave the best positive predictive value (84.8%) for pCR.21
Risk-of-Bias Assessment
Risks of bias was assessed for all included studies (n = 21) [ESM Fig. 1]. The individual risk-of-bias scores can be found in ESM Table 3 and ESM Table 4, on each risk of bias for each included study separately.
Discussion
Metabolic and immune biomarkers of the TME have a pivotal role in providing tumor cells the optimal condition to survive and proliferate while also influencing their response to therapy. Due to intratumoral and microenvironmental heterogeneity after nCRT, all available information from the tumor, its TME, and the pathological specimen was included. Here, we provide an overview of potential metabolic and immune TME biomarkers that might play a role in PR and CR after nCRT in EC.
Current research in targeting the metabolic TME is based on 18F-FDG-PET/CT imaging of the altered glycolytic tumor metabolism with acidification of the TME. TME acidification induces hypoxia response pathways and leads to evasion of the immune system, which is associated with high metastatic potential and treatment resistance.49 As such, the upregulation of glycolysis as a measure of extracellular acidification remains a critical step in the activation of immune cells. In this intricate interaction of heterogeneous tumor cells, a variety of secretory cytokines and chemokines from non-malignant cells, i.e., stroma and immune cells, are involved in the efficacy of anticancer therapy. Metabolic remodeling with inflammatory response and oxidative phosphorylation is important in the resistance to neoadjuvant treatment in EC. Recently, a promising novel ex vivo method showed the significance of oxidative phosphorylation in measuring real-time metabolic profiles of treatment-naïve EC biopsies. In clinical imaging of hypoxic response and glycolytic metabolism in malignant tumors, 18F-FDG-PET/CT is most commonly used.50 Based on the assessment of histopathology, the corresponding 18F-FDG-PET/CT response and promising biomarkers markers, nCRT combined with immunotherapy might be considered as an organ-preserving treatment approach in the near future.
Metabolic Tumor Microenvironment (TME) Markers
There were no studies on metabolic TME markers in EC that also assessed the influence of these markers on PR and/or CR after nCRT. Diabetes was suggested as a surrogate metabolic marker. However, the result of this study shows a limited role of DM on PR after nCRT. An overexpression of insulin receptors and insulin-like growth factors lead to the promotion of cell cycle progression and inhibition of apoptosis.51,52 The overexpressed insulin receptors on cancer cells of diabetic patients, who are also characterized by hyperinsulinemia, may be activated, leading to the ability of cancer cells to evade destruction by chemoradiotherapy, resulting in an unfavorable PR and CR.53 As a result, hypoxia and hyperglycemia occur, which might help remodeling the TME into an even more aggressive environment, leading to poorer response to nCRT.54
Immune TME Biomarkers
We showed that high CD3 and CD4 infiltration were generally correlated with better PR. Even though some studies showed no significant difference in CD8 between good and poor pathologic responders, CD8 infiltration in treatment-naïve biopsies was generally significantly associated with a better PR.24,27 One study showed that nCRT was useful to induce CD4 and CD8 infiltration within the TME, suggesting that an elevated level of lymphocytes before nCRT might be a surrogate of a strong immune response induced by tumor cell necrosis caused by chemotherapy.55
The activation of CD8 cells after nCRT might be impaired by persistent high expression of the CXCL12/CXCR4 axis in EC stem cells resulting in a downregulation of major histocompatibility complex (MHC) class I molecules and upregulating immunosuppressive cytokines.56 nCRT can also cause inflammation, leading to an influx of CD8 immune cells. These patients could benefit from the upcoming immune-directed treatment strategies such as PD-1/PD-L1 blockade.57,58,59,60 In this study, CD8 pre/post-nCRT and CD3/CD4 after nCRT seem to be involved in the antitumor response. Moreover, the location (i.e., tumoral or stromal) at which the CD8 influx occurs might affect active immune behavior. The extracellular matrix or other immune-suppressive cells within the tumor and the TME might barricade the function of tumoral CD8,61,62 resulting in an inefficient function of CD8 intratumorally.
The potential clinical value of tumoral PD-L1 expression in EC patients with residual disease after nCRT with surgery has shown to be significant for DFS after adjuvant anti-PD-1 nivolumab in the Checkmate-577 study, and showed a better PR in the Keynote-590 study with anti-PD-1 pembrolizumab and nCRT.3,7 The included studies also showed that a high proportion of PD-L1 in positive treatment-naïve tumor samples may affect PR; however, the exact mechanism behind this is still unknown. PD-L1 expression in pretreatment biopsies might be different due to intratumoral heterogeneity of EC, in which PD-L1 expression can only be partially captured. However, further investigation is needed.
We also showed that a higher expression of tumoral or stromal PD-L1 after nCRT is generally associated with a poor PR to chemoradiotherapy. Therefore, PD-L1 might be a potential target in EC patients receiving nCRT in order to improve therapy response. Together with the other predictive immune biomarkers, PD-L1 expression in the tumor and its microenvironment could be used to define EC patients with major or poor pathologic response after nCRT with resection and/or a clinical prognostic high- versus low-risk profile. PD-L1 positivity can be expressed by using both the tumor cell (TC ≥ 1% in at least 100 tumor cells in the PD-L1-stained slide) and combined positivity score (CPS ≥10 PD-L1-stained cells, including tumor cells, lymphocytes, macrophages in the associated infiltration). Based on the histologic EC subtypes, these clinical prognostic risk biomarkers and the different predictive response biomarkers between tumor-naïve biopsies and the resected residual tumor material potential biomarkers may be identified for the ypCR and non-ypCR groups.
18F-FDG-PET/CT Biomarkers
An 18F-FDG-PET/CT scan is commonly used in EC patients undergoing the CROSS regimen, to monitor treatment response. Many studies aimed to find a correlation between the semi-quantitative parameters of 18F-FDG-PET/CT with PR. However, our included studies showed contradictory evidence for the value of parameters such as SUV, ΔSUVmax and SUVmax in predicting PR and CR.
A low SUV might be associated with hypoxic tumors, as is the case in EC. An hypoxic environment could emerge if the tumor became more resistant to chemoradiotherapy, leading to a poor pathologic response.63 Moreover, a wide heterogeneity between studies could account for contradictory results, such as different methods and experience at performing and interpreting 18F-FDG-PET/CT scans, methods to calculate PET parameters, physiological factors that may affect SUV uptake (i.e., inflammation) to the esophageal mucosa, scanner technology, chemoradiotherapy schedules, sample size, and methods of data collection. Studies also vary regarding the time interval of post-treatment 18F-FDG-PET/CT after completion of nCRT, which may affect the interpretation of predictive accuracy.
Therefore, the predictive value of other clinical 18F-FDG-PET/CT-based markers needs to be explored. We showed that TLG and MTV might have more potential to predict pathological and clinical outcome, which is also in line with recent studies.64,65 These volume-based 18F-FDG-PET parameters might provide more valuable information that supplement SUV uptake for predicting PR and CR. Future studies should thus focus on combining these parameters and find a clear cut-off value.
The present study has some limitations. Treatment-naïve EC biopsies contain a highly heterogenous inflammatory secretion profile. It is plausible that pretreatment-naïve biopsies are not representative enough. Therefore, it is important to know which specimen has been used in determining the predictive role of biomarkers. First, tumor heterogeneity may be missed in these small standard diagnostic tumor biopsies, and second, we should be aware of changes in biomarkers during chemotherapy and/or radiotherapy.66 Furthermore, biology from resected tissue alone may not reflect tumor biology at diagnosis. Moreover, patients attaining pCR (ypT0/N0) who commonly exhibit a good prognosis will not likely receive adjuvant therapy. Furthermore, we included articles of various markers that were assessed in different ways, i.e., mRNA expression of assessed markers, assessments conducted in healthy esophageal mucosa, and overall density of assessed markers. These differences in assessing various markers made it difficult to interpret the results.
Conclusion
Our systematic review showed that CD8, CD4, CD3, and PD-L1 are promising immune markers in predicting PR. Moreover, we showed that TLG and MTV have potential in predicting CR and PR. Additional research should focus more on combining histopathology and nuclear imaging features in EC before and after nCRT to assess metabolic and immune TME markers.
References
Van Hagen P, Hulshof MCCM, Van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. https://doi.org/10.1056/NEJMoa1112088.
Faiz Z, Kats-Ugurlu G, Mui VEM, et al. Locoregional residual Esophageal cancer after Neo-Adjuvant Chemoradiotherapy and surgery regarding anatomic site and radiation target fields: a histopathologic evaluation study. Ann Surg. 2022;275(6):E759–65. https://doi.org/10.1097/SLA.0000000000004242.
Sun J-M, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–71. https://doi.org/10.1016/S0140-6736(21)01234-4.
Wang ZX, Cui C, Yao J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell. 2022;40(3):277-288.e3. https://doi.org/10.1016/j.ccell.2022.02.007.
Doki Y, Ajani JA, Kato K, et al. Nivolumab combination therapy in advanced Esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–62. https://doi.org/10.1056/nejmoa2111380.
Luo H, Lu J, Bai Y, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophaageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–25. https://doi.org/10.1001/jama.2021.12836.
Digklia A, Duran R, Homicsko K, et al. Cancer immunotherapy: a simple guide for interventional radiologists of new therapeutic approaches. Cardiovasc Intervent Radiol. 2019;42(9):1221–9. https://doi.org/10.1007/s00270-018-2074-1.
Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–203. https://doi.org/10.1056/nejmoa2032125.
Park S, Sun JM, Choi YL, et al. Adjuvant durvalumab for esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy: a placebo-controlled, randomized, double-blind, phase II study. ESMO Open. 2022;7(1):1–9. https://doi.org/10.1016/j.esmoop.2022.100385.
Shah MA, Kennedy EB, Alarcon-Rozas AE, et al. Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline. J Clin Oncol. 2023;41(7):1470–91. https://doi.org/10.1200/JCO.22.02331.
Mastracci L, Grillo F, Parente P, et al. PD-L1 evaluation in the gastrointestinal tract: from biological rationale to its clinical application. Pathologica. 2022;114(5):352–64. https://doi.org/10.32074/1591-951X-803.
Salem ME, Puccini A, Xiu J, et al. Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist. 2018;23(11):1319–27. https://doi.org/10.1634/theoncologist.2018-0143.
Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6(2):119–30. https://doi.org/10.21037/acs.2017.03.14.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. https://doi.org/10.1056/nejmoa1500596.
Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202(3):654–62. https://doi.org/10.1002/jcp.20166.
Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89(1):3–9. https://doi.org/10.1079/bjn2002763.
Fonteyne P, Casneuf V, Pauwels P, et al. Expression of hexokinases and glucose transporters in treated and untreated oesophageal adenocarcinoma. Histol Histopathol. 2009;24(8):971–7. https://doi.org/10.14670/HH-24.971.
Schreurs LMA, Smit JK, Pavlov K, et al. Prognostic impact of clinicopathological features and expression of biomarkers related to 18F-FDG Uptake in esophageal cancer. Ann Surg Oncol. 2014;21(12):3751–7. https://doi.org/10.1245/s10434-014-3848-6.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372. https://doi.org/10.1136/bmj.n71.
Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:4–10. https://doi.org/10.1136/bmj.i4919.
Li C, Lin J-W, Yeh H-L, Chuang C-Y, Chen C-C. Good prediction of treatment responses to neoadjuvant chemoradiotherapy for esophageal cancer based on preoperative inflammatory status and tumor glucose metabolism. Sci Rep. 2021;11(1):11626. https://doi.org/10.1038/s41598-021-90753-y.
Wang J, Myles B, Wei C, et al. Obesity and outcomes in patients treated with chemoradiotherapy for esophageal carcinoma. Dis Esophagus. 2014;27(2):168–75. https://doi.org/10.1111/dote.12074.
Fassan M, Cavallin F, Guzzardo V, et al. PD-L1 expression, CD8+ and CD4+ lymphocyte rate are predictive of pathological complete response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic esophagus. Cancer Med. 2019;8(13):6036–48. https://doi.org/10.1002/cam4.2359.
Goedegebuure RSA, Harrasser M, de Klerk LK, et al. Pre-treatment tumor-infiltrating T cells influence response to neoadjuvant chemoradiotherapy in esophageal adenocarcinoma. Oncoimmunology. 2021;10(1):1954807. https://doi.org/10.1080/2162402X.2021.1954807.
Huang W-T, Lu H-I, Wang Y-M, et al. Positive Programmed Cell Death-Ligand 1 Expression Predicts Poor Treatment Outcomes in Esophageal Squamous Cell Carcinoma Patients Receiving Neoadjuvant Chemoradiotherapy. J Clin Med. 2019;8(11). doi:https://doi.org/10.3390/jcm8111864
Kotsafti A, Scarpa M, Cavallin F, et al. Immune surveillance activation after neoadjuvant therapy for esophageal adenocarcinoma and complete response. Oncoimmunology. 2020;9(1):1804169. https://doi.org/10.1080/2162402X.2020.1804169.
Soeratram TT, Creemers A, Meijer SL, et al. Tumor-immune landscape patterns before and after chemoradiation in resectable esophageal adenocarcinomas. J Pathol. 2022;256(3):282–96. https://doi.org/10.1002/path.5832.
Göbel HH, Distel LVR, Aigner T, Büttner-Herold MJ, Grabenbauer GG. PD-1 and PD-L1 expression predict regression and prognosis following neoadjuvant radiochemotherapy of oesophageal adenocarcinoma. Clin Transl Radiat Oncol. 2022;34:90–8. https://doi.org/10.1016/j.ctro.2022.04.001.
Haddad R, Zlotnik O, Goshen-lago T, et al. Tumor lymphocyte infiltration is correlated with a favorable tumor regression grade after neoadjuvant treatment for esophageal adenocarcinoma. J Pers Med. 2022;12(4):627. https://doi.org/10.3390/jpm12040627.
Koemans WJ, Van Dieren JM, Den Van Berg JG, et al. High CD8 tumour-infiltrating lymphocyte density associates with unfavourable prognosis in oesophageal adenocarcinoma following poor response to neoadjuvant chemoradiotherapy. Histopathology. 2021;79:238–51. https://doi.org/10.1111/his.14361.
Alvarado CE, Kapcio KC, Lada MJ, Linden PA, Towe CW, Worrell SG. The effect of diabetes on pathologic complete response among patients with esophageal cancer. Semin Thorac Cardiovasc Surg. 2023;35(2):429–36. https://doi.org/10.1053/j.semtcvs.2021.10.016.
Fang P, Musall B, Ma J, et al. Interim FDG-pet imaging as an early response biomarker for pathologic response to chemoradiation in esophageal cancer. Int J Radiat Oncol Biol Phys. 2017;99(2):S14–5. https://doi.org/10.1016/j.ijrobp.2017.06.050.
Gillham CM, Lucey JA, Keogan M, et al. 18FDG uptake during induction chemoradiation for oesophageal cancer fails to predict histomorphological tumour response. Br J Cancer. 2006;95(9):1174–9. https://doi.org/10.1038/sj.bjc.6603412.
Kukar M, Alnaji RM, Jabi F, et al. Role of repeat 18F-fluorodeoxyglucose positron emission tomography examination in predicting pathologic response following neoadjuvant chemoradiotherapy for esophageal adenocarcinoma. JAMA Surg. 2015;150(6):555–62. https://doi.org/10.1001/jamasurg.2014.3867.
Arnett ALH, Merrell KW, Martin Macintosh EL, et al. Utility of [18F]FDG positron emission tomography for predicting histopathologic response in esophageal carcinoma following chemoradiation. Int J Radiat Oncol. 2016;96(2):E139. https://doi.org/10.1016/j.ijrobp.2016.06.940.
Choi Y, Choi JY, Hong TH, et al. Trimodality therapy for locally advanced esophageal squamous cell carcinoma: the role of volume-based PET/CT in patient management and prognostication. Eur J Nucl Med Mol Imaging. 2022;49(2):751–62. https://doi.org/10.1007/s00259-021-05487-w.
Dewan A, Sharma SK, Dewan AK, et al. Impact on Radiological and Pathological Response with Neoadjuvant Chemoradiation and Its Effect on Survival in Squamous Cell Carcinoma of Thoracic Esophagus. J Gastrointest Cancer. 2017;48(1):42–9. https://doi.org/10.1007/s12029-016-9870-0.
Lee J, Choi JY, Lim SW, et al. Utility of positron emission–computed tomography for predicting pathological response in resectable oesophageal squamous cell carcinoma after neoadjuvant chemoradiation. Eur J Cardio-thoracic Surg. 2021;58(5):1019–26. https://doi.org/10.1093/EJCTS/EZAA181.
Piessen G, Petyt G, Duhamel A, Mirabel X, Huglo D, Mariette C. Ineffectiveness of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of tumor response after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg. 2013;258(1):66–76. https://doi.org/10.1097/SLA.0b013e31828676c4.
van Rossum PSN, Fried DV, Zhang L, et al. The value of (18)F-FDG PET before and after induction chemotherapy for the early prediction of a poor pathologic response to subsequent preoperative chemoradiotherapy in oesophageal adenocarcinoma. Eur J Nucl Med Mol Imaging. 2017;44(1):71–80. https://doi.org/10.1007/s00259-016-3478-2.
Boyd JK, Jones DR, Kozower B, Lau C, Sanoff HK. The impact of diabetes mellitus and obesity on pathologic complete response rate of neoadjuvant chemoradation for gastroesophageal cancer: a retrospective review. J Clin Oncol. 2010;28(15 Suppl):e14627. https://doi.org/10.1200/JCO.2010.28.15_SUPPL.E14627.
Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: Implications for response classification. Ann Surg. 2005;242(5):684–92. https://doi.org/10.1097/01.sla.0000186170.38348.7b.
Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103(7):1347–55. https://doi.org/10.1002/cncr.20916.
Fassan M, Cavallin F, Guzzardo V, et al. CD4+ and CD8+ lymphocyte rate and PDL-1 leukocyte expression are predictive of clinical complete response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus. United Eur Gastroenterol J. 2017;5(5):A589. https://doi.org/10.1177/2050640617725676.
Goedegebuure RSA, De Klerk LK, Bass AJ, Derks S, Thijssen VLJL. Combining radiotherapy with anti-angiogenic therapy and immunotherapy; A therapeutic triad for cancer? Front Immunol. 2019;10(Jan):1-15. doi:https://doi.org/10.3389/fimmu.2018.03107
Haddad R, Zlotnik O, Goshen-lago T, et al. Tumor lymphocyte infiltration is correlated with a favorable tumor regression grade after neoadjuvant treatment for esophageal adenocarcinoma. J Pers Med. 2022;12(4):626. https://doi.org/10.3390/jpm12040627.
Arnett ALH, Merrell KW, Macintosh EM, et al. Utility of (18)F-FDG PET for predicting histopathologic response in esophageal carcinoma following chemoradiation. J Thorac Oncol. 2017;12(1):121–8. https://doi.org/10.1016/j.jtho.2016.08.136.
Piessen G, Robb WB, Petyt G, et al. Ineffectiveness of 18f-fluorodeoxyglucose positron emission tomography in the evaluation of tumour response after completion of neoadjuvant chemoradiation in oesophageal cancer. Eur Surg Res. 2013;50(2):195–6. https://doi.org/10.1159/000351963.
Barsoum IB, Koti M, Siemens DR, Graham CH. Mechanisms of hypoxia-mediated immune escape in cancer. Cancer Res. 2014;74(24):7185–90. https://doi.org/10.1158/0008-5472.CAN-14-2598.
Buckley AM, Dunne MR, Morrissey ME, et al. Real-time metabolic profiling of oesophageal tumours reveals an altered metabolic phenotype to different oxygen tensions and to treatment with Pyrazinib. Sci Rep. 2020;10(1):12105. https://doi.org/10.1038/s41598-020-68777-7.
Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35. https://doi.org/10.1016/j.metabol.2018.11.001.
Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. https://doi.org/10.1038/nrc1408.
Armengol-Alonso A, Tusquets I, Servitja S, et al. Association between insulin resistance (Ir) and pathologic complete response (Pcr) to neoadjuvant chemotherapy (Nac) among no diabetic women with localized infiltrating breast cancer (Libc). Ann Oncol. 2014;25(Suppl 4):iv114. https://doi.org/10.1093/annonc/mdu328.13.
Rojas A, Lindner C, Schneider I, et al. Diabetes mellitus contribution to the remodeling of the tumor microenvironment in gastric cancer. World J Gastrointest Oncol. 2021;13(12):1997–2012. https://doi.org/10.4251/wjgo.v13.i12.1997.
Tsuchikawa T, Miyamoto M, Yamamura Y, Shichinohe T, Hirano S, Kondo S. The immunological impact of neoadjuvant chemotherapy on the tumor microenvironment of esophageal squamous cell carcinoma. Ann Surg Oncol. 2012;19(5):1713–9. https://doi.org/10.1245/s10434-011-1906-x.
Wu X, Zhang H, Sui Z, Wang Y, Yu Z. The biological role of the CXCL12/CXCR4 axis in esophageal squamous cell carcinoma. Cancer Biol Med. 2021;18(2):401–10. https://doi.org/10.20892/j.issn.2095-3941.2020.0140.
Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27(20):3297–302. https://doi.org/10.1200/JCO.2009.19.6410.
Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71. https://doi.org/10.1016/S0140-6736(17)31827-5.
Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (Anti–PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. https://doi.org/10.1056/nejmoa1305133.
Bang YJ, Yañez Ruiz E, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052–60. https://doi.org/10.1093/annonc/mdy264.
Bercovici N, Guérin MV, Trautmann A, Donnadieu E. The remarkable plasticity of macrophages: a chance to fight cancer. Front Immunol. 2019;10(JULY):1–9. https://doi.org/10.3389/fimmu.2019.01563.
Carstens JL, De Sampaio PC, Yang D, et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun. 2017;8:15095. https://doi.org/10.1038/ncomms15095.
Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26(2):225–39. https://doi.org/10.1007/s10555-007-9055-1.
Tamandl D, Ta J, Schmid R, et al. Prognostic value of volumetric PET parameters in unresectable and metastatic esophageal cancer. Eur J Radiol. 2016;85(3):540–5. https://doi.org/10.1016/j.ejrad.2016.01.002.
Malik V, Johnston C, O’Toole D, et al. Metabolic tumor volume provides complementary prognostic information to EUS staging in esophageal and junctional cancer. Dis Esophagus. 2017;30(3):1–8. https://doi.org/10.1111/dote.12505.
Shin J, Chung JH, Kim SH, et al. Effect of platinum-based chemotherapy on PD-L1 expression on tumor cells in non-small cell lung cancer. Cancer Res Treat. 2019;51(3):1086–97. https://doi.org/10.4143/crt.2018.537.
Funding
No external funding was secured for this study. Helena H. Wang, Emelie N. Steffens, Gursah Kats-Ugurlu, Boudewijn van Etten, Johannes G.M. Burgerhof, Geke A.P. Hospers, and John T.M. Plukker have no financial relationships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Conflicts of interest to disclose that are relevant to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H.H., Steffens, E.N., Kats-Ugurlu, G. et al. Potential Predictive Immune and Metabolic Biomarkers of Tumor Microenvironment Regarding Pathological and Clinical Response in Esophageal Cancer After Neoadjuvant Chemoradiotherapy: A Systematic Review. Ann Surg Oncol 31, 433–451 (2024). https://doi.org/10.1245/s10434-023-14352-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14352-z